General Anatomy
Anatomy – Reveal the Concealed
What Anatomy Is
“ανατoμη” (anatome) means cut-up, “ανατεμνειν” (anatemnein) denotes to cut open. Consequently, anatomy is dissection and the anatomists are dissectors. The dissection reveals the otherwise non-visible constituents, and is the method which named the science: reveal, represent, divide, cut, sort, and name. Recognition of the parts is the key to understanding the subject.
“Anatomy […] dissects organisms into their […] constituents […], examining their external, sensorial perceptible properties and their internal structure. It is the study of death to make conclusions about life. Anatomy manually destroys an ideal creation in order to rebuild it mentally and to virtually recreate a human being. There is not a more glamorous task for the human mind.”
Joseph Hyrtl (Anatomist, 1811–1894).
Although anatomy deals with death, it is devoted to life. It is not about death but rather about the comprehension of the human body which functions as a unit. The body donors are models only.
There are two other medical fields which deal with dead bodies: forensic medicine and pathology. Pathologists are interested in causes of diseases. Forensic medicine deals in particular with doubtful causes of death. Whereas the sole purpose of anatomists is to understand the living human body on a continuum from the embryonic stage to old age.
Eyes and hands are most important tools of the anatomist. The findings revealed by hands, tweezers, scissors, scalpels, and the visualization of these structures by eye is called gross or macroscopic anatomy. Structures in gross anatomy not discernible by the naked eye can be visualized by microtomes or light and electron microscopes. This field is called microscopic anatomy.
Organization and classification are basic aspects of systematic anatomy. The body is precisely classified according to systems. The bone system for example includes not only bones, but also bony parts and associated terminology. On the other hand, tissue systems are organized according to types and subtypes. Topographic anatomy is the study of regions or divisions of the body and emphasizes the relations between various structures in that region. The relationship of form and function is termed functional anatomy. Topographic anatomy and functional anatomy are the supreme disciplines of the physician and lead the path to clinical anatomy. This serves as practical application for diagnosis and therapy. Lastly, comparative anatomy serves in evolutionary phylogeny. It is of interest to biologists and compares bodies and body parts of different creatures.
Histology is a subdivision of microscopic anatomy and is dealing with the composition of organ tissues which are multicellular in structure. Cytology, the study of cells, focuses on structure and function of the single cell. Embryology, which mainly uses the microscope for examination of tiny embryos, describes the development of an organism (individual development, ontogenesis).
Dissection and analysis is the trade of the anatomy, but its real goal is to mentally assemble all parts into a functioning whole. This goal of understanding the structural design and shape of biological structures and conceptualizing it as a unified structure-function relationship can also be called morphology.
Linguae Anatomiae
The language of this classical discipline “Anatomy” (Linguae anatomiae) is predominantly Latin and (latinized) Greek. In the past 50 years, some English terms were added. The anatomic Termini technici (terminology) are usually marvellously graphic, concrete, and vivid. Even a word monster like “Cartilago arytenoidea” means simply (nothing more than) “the cartilage which looks like a gravy boat”. This cartilage is located above the larynx and really looks like a boat-shaped pitcher to serve gravy. At times one needs visual imagination which anatomists do not lack. One does not need to be afraid of terminology, but rather enjoy its diversity. This is done most successfully when this terminology is translated into one’s own language and imagination.
Body Donations – The Legacy
Dead human bodies are essential for carrying out lessons in dissection. These bodies are made available by body donations. The body donor bequeathed his/her body to an anatomical institute. This has to be done in person as a last will declaration during the lifetime of the donor. Next of kin are not authorized representatives in this legal matter. Every body donor has personally contacted an anatomy institute during his/her lifetime and, in the last will, donated his/her body to the institution for teaching and research after death.
The body donor usually receives a donor card which always needs to be at hand. When death occurs the body is brought to the anatomy institute and is used for lessons in dissection, for clinical preparations, for demonstration, or for surgery courses as well as for scientific studies.
Following the courses and examinations, the mortal remains are usually cremated and buried in the cemetery of honour of the university. The memorial or funeral service is attended by family members, students, and instructors of the faculty.
Depending on institution and/or state/province, there are different regulations for the exhibition of bodies and organs. For example, body donors or organs of body donors can be exhibited in an anatomical collection for presentation and teaching purposes, if this is expressed in the body donor’s will.
Reasons for body donations are diverse, and body donors represent all parts of society. The widely held assumption that body donors donate to be granted an inexpensive funeral is proven to be wrong. Many universities charge a fee for body donations and this has not resulted in a reduction of body donations.
→Dissection Link
Dissection is done by hand using a scalpel (non-disposable scalpels!) and anatomical tweezers. Structures and organs as well as their topographic relationships are examined in this fashion.
The nature of the tissue differs regionally. Areas with a lot of adipose tissues that can be removed bluntly by hand alternate with connective tissue which can be stripped off with the aid of scalpel only. As part of the preparation, different cavities are exposed which are filled with air, liquid, or solid constituents. The tissue of the organs (parenchyma) may – depending on the fixation – be hard, soft, spongy, tender, or elastic. Protected nerves and blood vessels are located in different layers of the body, and their dissection can be of varying difficulty. In some locations these are easily removable, in other regions they may adhere to adjacent tissues. To illustrate the muscles, mobilization by loosening the tight surrounding connective tissue sheaths (muscle fascia) is required.
To prevent damage, special attention needs to be paid to nerves and blood vessels entering and exiting the muscle. Partial severance of surrounding ligaments is needed to open joints. In contrast some structures such as the inner ear can be exposed with a hammer and chisel or saws and milling machines.
The preparation requires a lot of patience, manual dexterity, and spatial imagination. One gains great experiences and valuable insights which are not offered by any anatomy textbook or atlas. These include the three-dimensional understanding of the structures of the human body, the confrontation with death, but also teamwork.
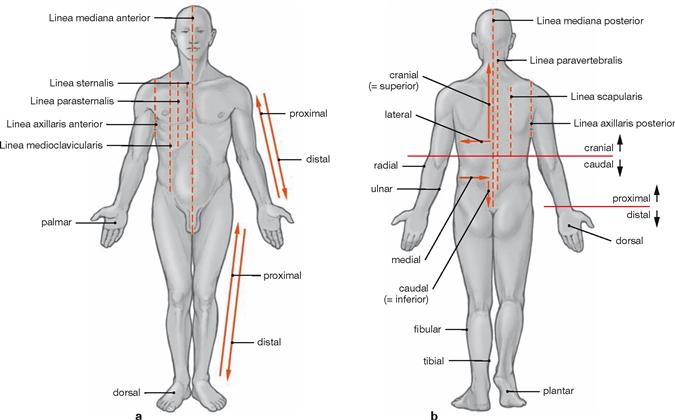
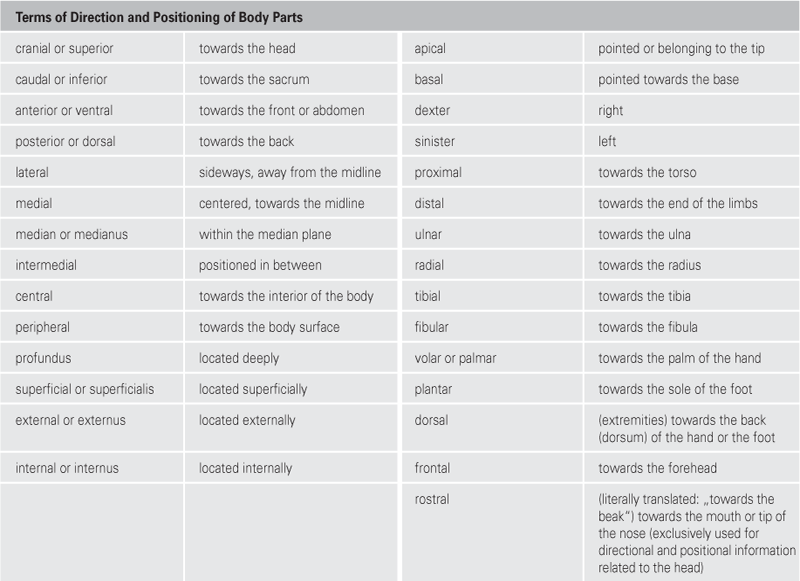
Orientation on the body
Axes and planes
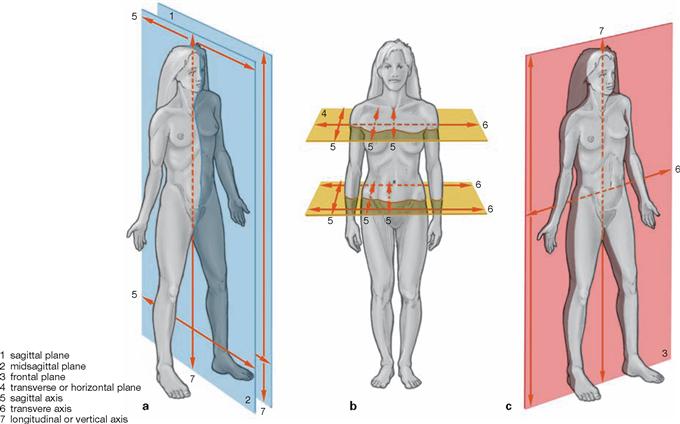
| Main Axes | |
| sagittal axis | is positioned perpendicular to transverse and longitudinal axis |
| transverse axis | is positioned perpendicular to longitudinal and sagittal axis |
| longitudinal or vertical axis | is positioned perpendicular to sagittal and transverse axis |
| Main Planes | |
| median (sagittal) plane | symmetry plane, divides the body into two equal halves |
| sagittal plane | runs parallel to the median (sagittal) plane |
| transverse plane | any cross-sectional plane of the body |
| frontal plane | parallel to the forehead |
| Radiological Section Planes | |
| Radiological Terms | Anatomical Terms |
| sagittal section | sagittal plane |
| coronal section | frontal plane |
| axial section | transverse plane |
Radiology terminology in imaging procedures (computed tomography and magnetic resonance imaging) defines the three main anatomical planes as sections with their own nomenclature.
Surface anatomy
Parts of the body
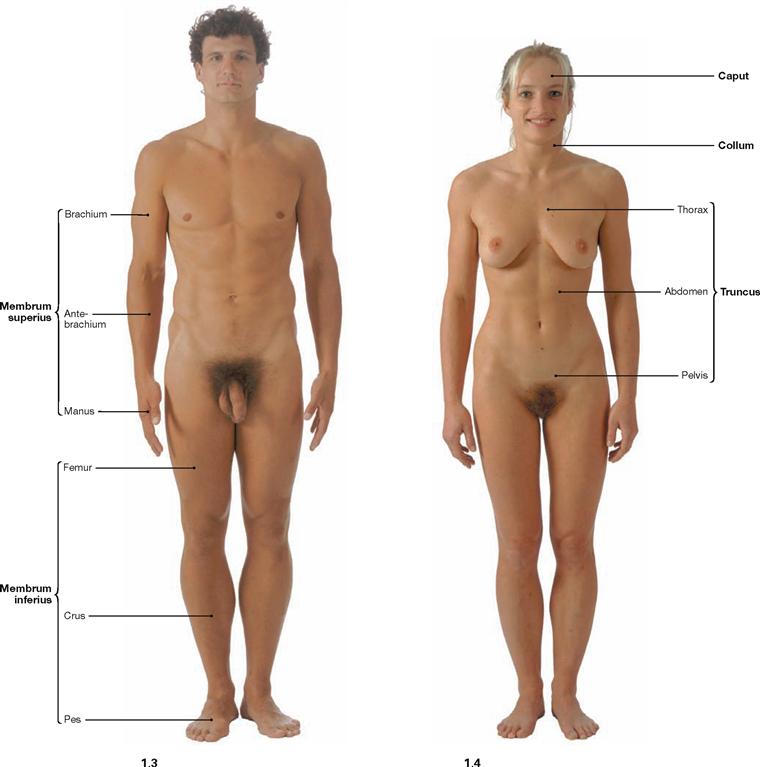
Fig. 1.3 and Fig. 1.4 Surface anatomy of the male (→ Fig. 1.3) and the female (→ Fig. 1.4); ventral view.
Anatomical terminology generally refers to the upright position with the face directed forwarzd, arms positioned sideways, palms pointing towards the body or forward, legs positioned beside each other with feet pointing forward.
The body is divided into head (Caput), neck (Collum), torso (Truncus) with chest (Thorax), abdomen (Abdomen), pelvis (Pelvis), back (Dorsum), and upper (Membrum superius) and lower (Membrum inferius) extremities. The extremities divide into the upper arm (Brachium), forearm (Antebrachium), hand (Manus) and upper leg (Femur), lower leg (Crus), foot (Pes).
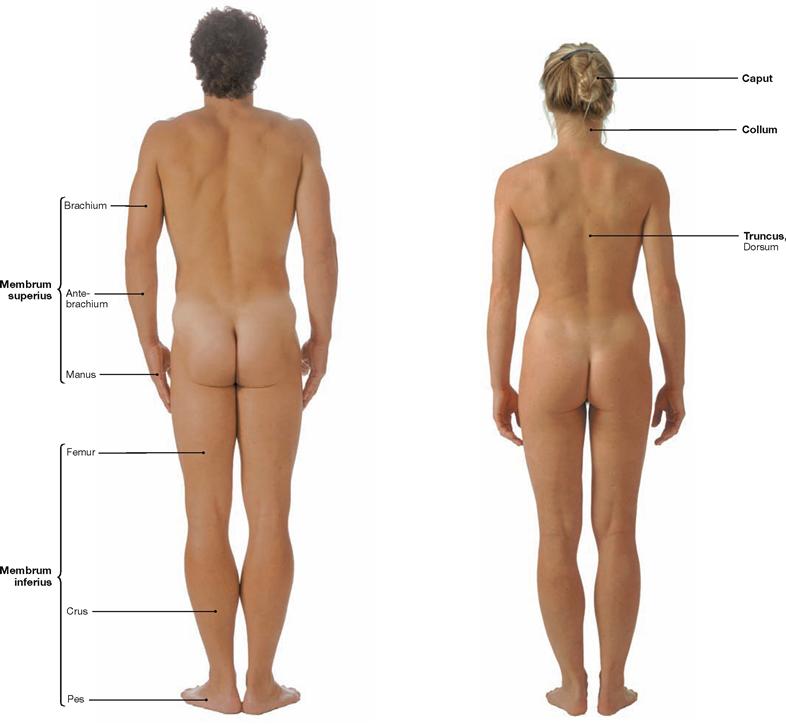
Fig. 1.5 and Fig. 1.6 Surface anatomy of the male (→ Fig. 1.5) and female (→ Fig. 1.6); dorsal view.
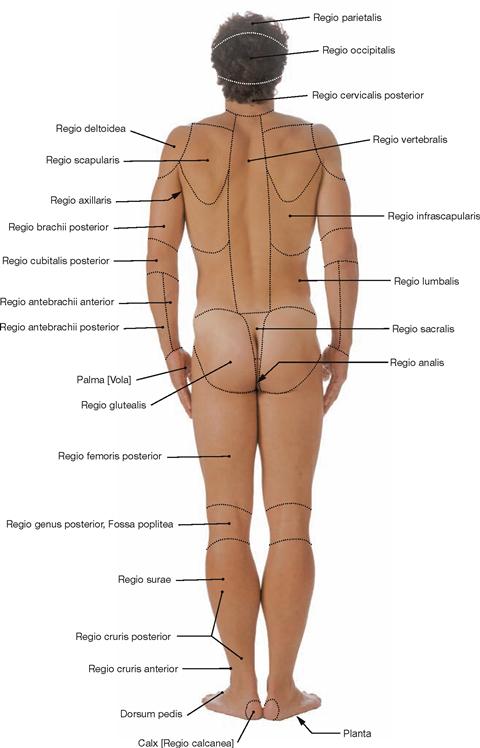
Regions of the body
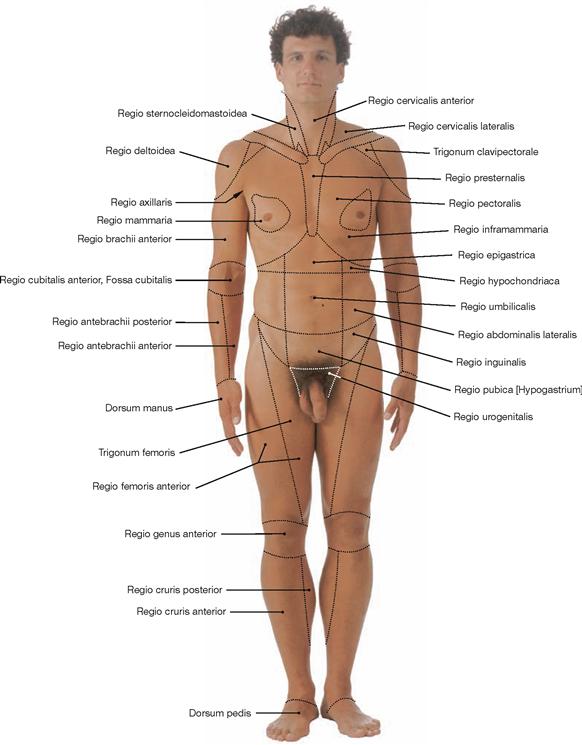
Fig. 1.7 Body regions; ventral view.
The body surface is divided into regions for better description and orientation. Regio: region; Trigonum: triangle.
Inner organs, surface projection
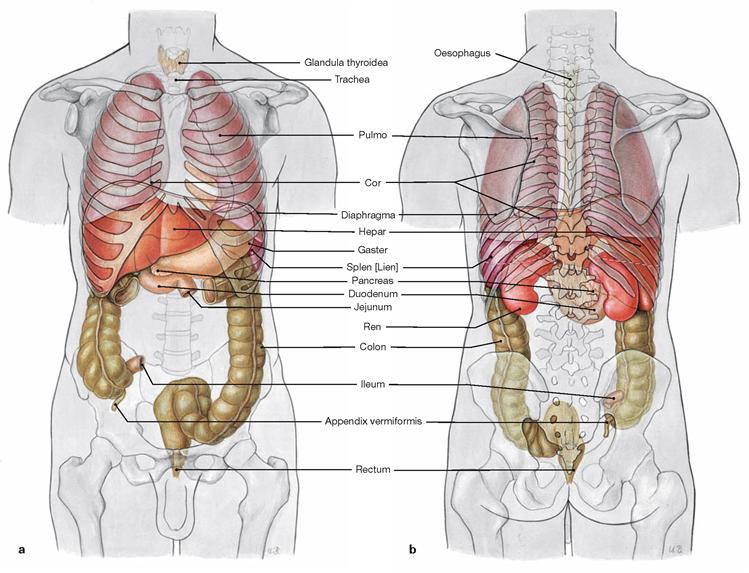
Figs. 1.9a and b Projection of inner organs onto the body surface. Projection of inner organs onto the ventral abdominal wall (a) and onto the dorsal wall of the trunk (b): esophagus, thyroid gland (Glandula thyroidea), wind pipe (Trachea), lung (Pulmo), heart (Cor), diaphragm, liver (Hepar), stomach (Gaster), spleen (Splen [Lien]), pancreas, duodenum, jejunum, kidney (Ren), colon, ileum, appendix (Appendix vermiformis), and rectum (Rectum).
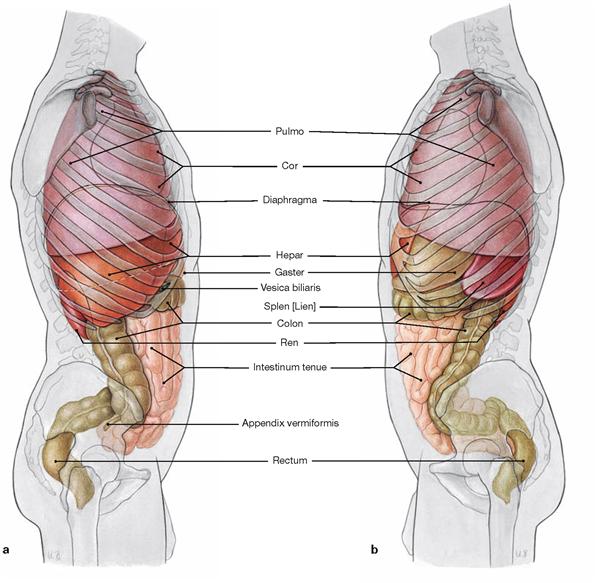
Figs. 1.10a and b Projection of inner organs onto the body surface. Projection of inner organs onto the right wall of the torso (a) and onto the left wall of the torso (b): lung (Pulmo), heart (Cor), diaphragm, liver (Hepar), stomach (Gaster), gall bladder (Vesica biliaris), spleen (Splen [Lien]), colon, kidney (Ren), small intestine (Intestinum tenue), appendix (Appendix vermiformis), and rectum.
Development
Development
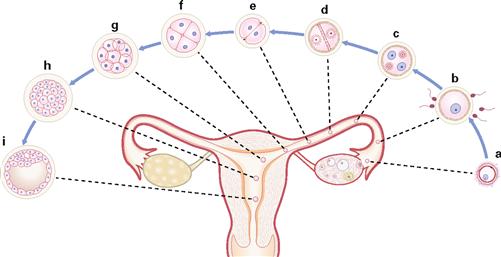
Figs. 1.11a to i First week of embryogenesis: fertilization and implantation. [21]
Within 24 hours after ovulation (a), fertilization (b) normally occurs in the ampulla of the oviduct. The fusion of the pronuclei of the ovum and sperm into a single diploid nucleus creates the zygote
(c). Subsequent cell divisions (2-, 4-, 8- and 16-cell stages; d–h) gen-erate a cell aggregate (Morula) which is transported into the uterine cavity. At approximately day 5 after fertilization, the morula develops into a fluid-filled cyst (blastocyst; i) which implants into the uterine mucosa at days 5–6.
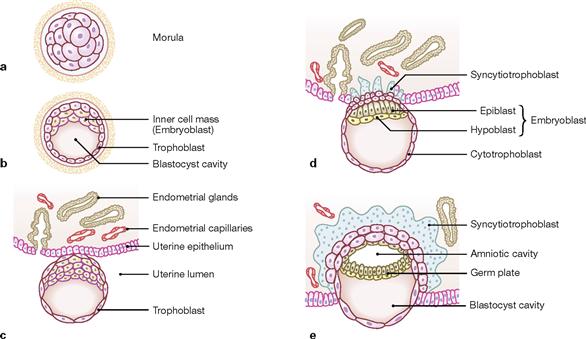
Figs. 1.12a to e First and second week of embryogenesis: bilaminar embryonic disc. [21]
Upon differentiation of the morula (a) into the blastocyst, the latter generates an inner cell mass (embryoblast) and a larger fluid-filled (blastocyst cavity) outer cell layer (trophoblast; b). Through interactions between maternal tissues and the trophoblast cells the utero-placental circulation is formed (c–e). The embryoblast develops into the bilaminar embryonic disc with ectoderm (columnar cells at the dorsal surface of the embryoblast) and entoderm (cuboidal cells at the ventral surface). The ectoderm forms a dorsally located cavity which becomes the amniotic cavity. The ventrally located blastocyst cavity becomes the primary yolk sac which is lined by entoderm. At day 12, the secondary yolk sac (yolk sac proper) forms. The original blastocyst cavity is lined by extra-embryonic mesoderm.
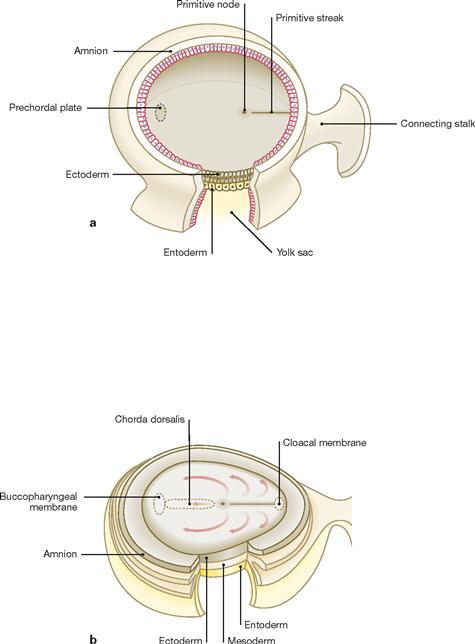
Figs. 1.13a and b Third week of embryogenesis: gastrulation. [21]
Development of the trilaminate embryonic disc initiates with the appearance of the primitive streak at the dorsal surface of the ectoderm. At its cranial section, the primitive streak is demarcated by the primitive node (a). Cells migrating out of the primitive streak form the intraembryonic mesoderm located between the top of the yolk sac and the ectoderm of the amniotic cavity (gastrulation). Some of these cells form the notochordal process which extends towards the cranial part of the embryo where the prechordal plate has formed (adhesion between ectoderm and entoderm without an intervening mesoderm layer). The notochordal process develops a lumen (notochordal canal) and becomes the notochord (Chorda dorsalis; primitive stabilizing structure of the embryo) which regresses later in development (b). Relics of the notochord can be found in the Nuclei pulposi located within the vetrebral discs. Some mesoderm cells migrate cranially past the prechordal plate to create the primordial heart. The three germ layers (ectoderm, mesoderm, entoderm) are the building blocks for the development of all organs. Further information on the germ layers participating in specific organ formation can be found in embryology textbooks.
Skeleton
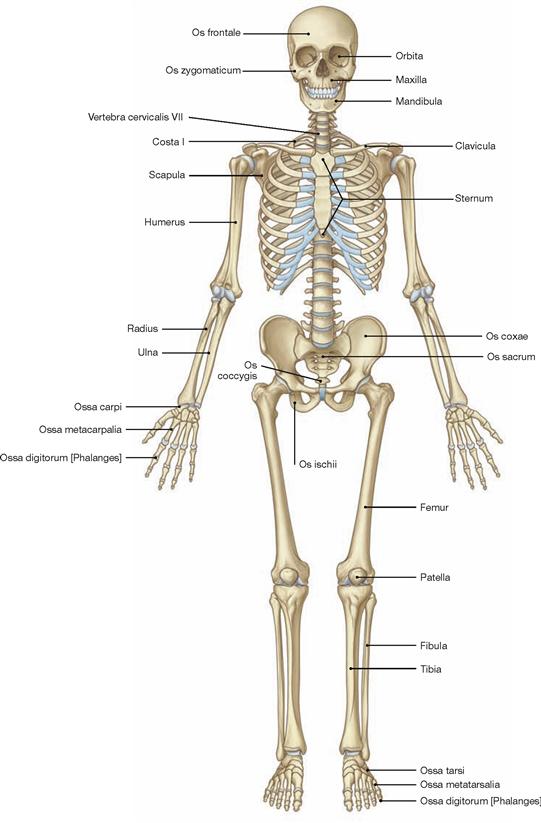
Fig. 1.14 Skeleton, Systema skeletale; ventral view. [10]
The bones of the skeleton are grouped according to their shape and structure:
• long bones (Ossa longa), e.g. hollow bones of the extremities, like femur and humerus
• short bones (Ossa brevia), e.g. carpal and tarsal bones
• flat bones (Ossa plana), e.g. ribs, sternum, scapula, pelvis, bones of the skull
• air-filled bones (Ossa pneumatica), e.g. frontal bone, ethmoid bone, maxilla, sphenoid bone
• irregular bones (Ossa irregularia, cannot be grouped with the other bones), e.g. vertebrae, mandible
• sesamoid bones (Ossa sesamoidea, bones embedded in tendons), e.g. patella, Os piriformis
• accessory bones (Ossa accessoria, accessory bones not commonly found in all human skeletons), e.g. sutural bones of the skull, cervical rib
Musculoskeletal system
Structure of bones
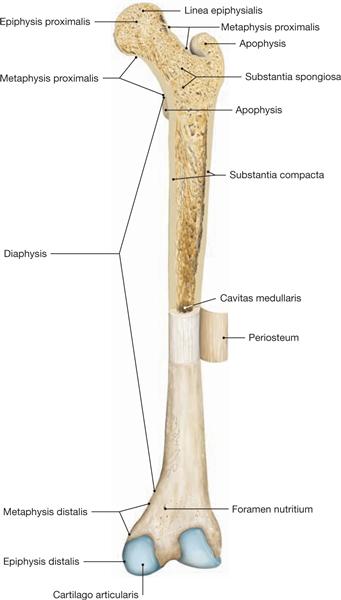
Fig. 1.15 Long bones (hollow bones), Os longum.
Section through the proximal part of the femoral bone of an adult. Periosteum of the diaphysis has been removed and folded sideways. Dorsal view. Sectioned femoral bone displays two distinct types of bones with no clear separation between them:
• Substantia compacta or corticalis (compacta, compact bone, very thin in the epiphysis, substantial in the diaphysis) and
• Substantia spongiosa (spongiosa, spongy or cancellous bone, substantial presence exclusively in the epiphysis and metaphysis).
In the diaphysis, the compacta appears as a solid mass; the spongiosa in epi- and metaphysis creates a three-dimensional network of delicate branched bones (trabeculae). Depending on the physical forces applying, they are divided into traction or compression trabeculae. The space in between the trabeculae is filled with blood-forming bone marrow (young person) or fatty lipids (old person). The orientation of the individual trabeculae is parallel to the lines of tensile and compressive stress generated within the bone. (In the femur, these forces are proximal and eccentric, adding additional bending stress to the bone.) A long evolutionary process resulted in a light bone, combining maximal mechanical robustness with minimal bone deposit.
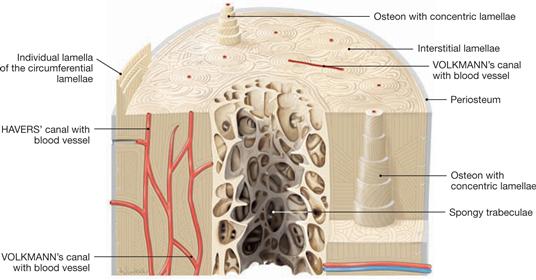
Fig. 1.16 Structure of a long hollow bone, Os longum.
The basic histological structure of both a mature compact bone and a mature spongy bone is similar and represents a lamellar bone. The mature bone is composed of lamellar concentric units, named osteons, most frequently found in the compacta of long bones. In spongy bones, the lamellae are primarily oriented parallel to the trabecular surface. In the compact bone, lamellae of bone matrix with central blood vessels create osteons, a system (HAVERS’ system) of five to 20 bony lamellae (special lamellae) which are grouped concentrically around a HAVERS’ canal and can be a few centimeters in length. Collagen fibres show perpendicular orientation in adjacent lamellae of an osteon. Remnants of previous osteons, called interstitial lamellae, are located between osteons. The outer and inner surface of the compacta is composed of lamellae surrounding the complete bone. These are called outer and inner circumferential lamellae.
Bone development
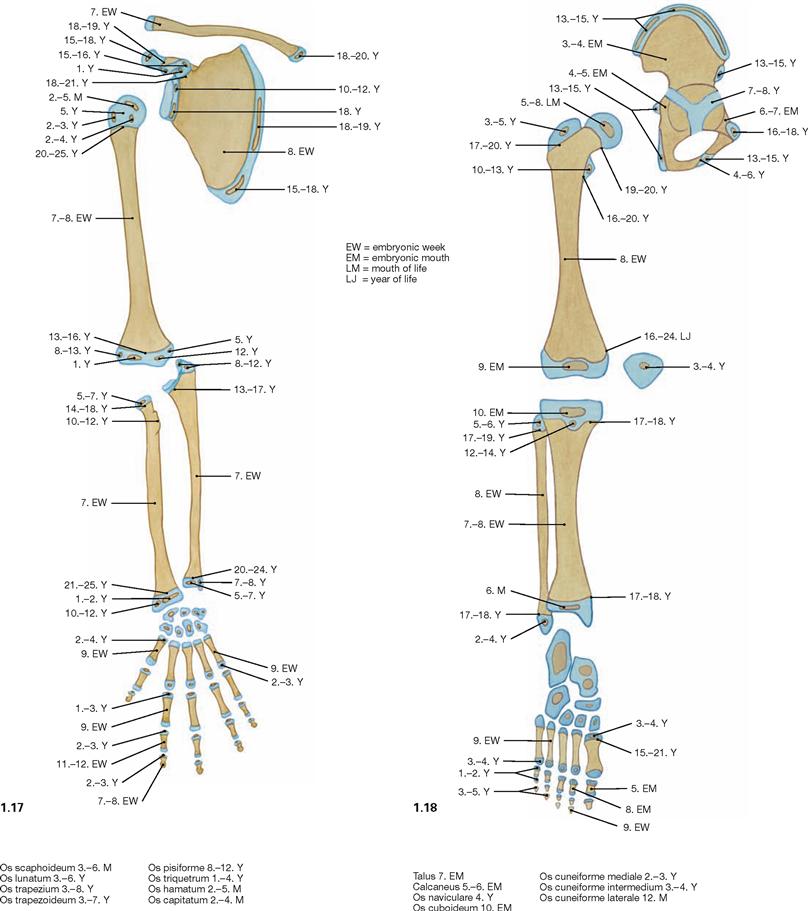
Fig. 1.17 and Fig. 1.18 Ossification of the skeleton of the upper (→ Fig. 1.17) and lower extremities (→ Fig. 1.18); position of the epi- and apophysial ossification centres and chronological sequence of the formation of these ossification centres.
The timing for these bone nucleation sites to appear holds clues as to the stage reached in skeletal development and, thus, to the individual skeletal and bone age. We distinguish ossification centres formed around the shaft (diaphysis) of the cartilage model during the fetal period, resulting in the diaphyses (diaphyseal ossification) from ossification centres which in part form during the second half of the fetal period and in the first years of life within the cartilaginous epi- and apophyses (epi- and apophyseal ossification). No further increase in body height occurs once the cartilaginous epiphyseal gaps ossify and disappear (synostosis). Thereafter, isolated bone nucleation sites are no more visible in the X-ray image.
Joints
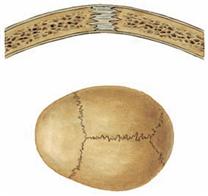
Fig. 1.19 Fibrous joint, Junctura fibrosa [Syndesmosis].
Fibrous joints between bones are found in sutures of the skull, syndesmoses (e.g. fibrous connections between the tibia and fibula or the radius and ulna), and gomphoses (e.g. fibrous anchoring of the teeth in their alveolar sockets of the maxilla and mandibula).
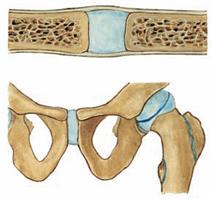
Fig. 1.20 Cartilaginous joint, Junctura cartilaginea [Synchondrosis].
Cartilaginous joints connect bones through hyaline cartilage (synchondrosis, e.g. connection between 1. rib and clavicle) or fibrocartilage (symphysis, e.g. Symphysis pubica).
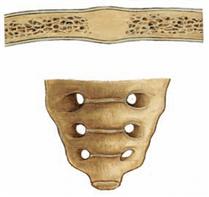
Fig. 1.21 Osseous joint, Junctura ossea [Synostosis].
At the osseous joints bones are fused as exemplified by the sacrum.
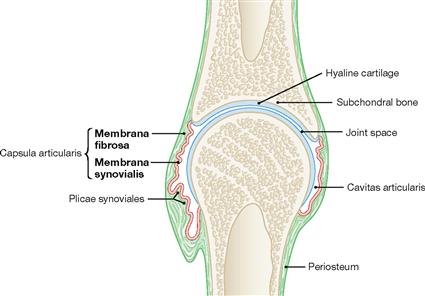
Fig. 1.22 Synovial (true) joint, Junctura synovialis [Articulatio synovialis, Diarthrosis]; schematic sectional view. (according to [1])
Hyaline cartilage at the bony ends covers the subchondral bone. The joint capsule encloses the joint cavity and consists of an outer fibrous membrane (Membrana fibrosa) and an innner synovial membrane (Membrana synovialis). The synovial membrane secretes the synovia into the joint cavity which acts as the grease of the joint. When the freedom of motion of a joint is restricted by an exceptionally strong joint capsule, this joint is called amphiarthrosis (e.g. small carpal joints of the hand and foot; Articulatio sacroiliaca).
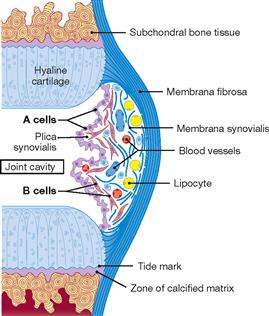
Fig. 1.23 Structure of the joint capsule. [24]
The joint capsule is composed of the Membrana fibrosa and the Membrana synovialis. The Membrana fibrosa consists of tough fibrous tissue. The Membrana synovialis is composed of the following layers: a superficial loose layer of A cells (type A synovialocytes or M cells, specialized macrophages which metabolize the metabolic compounds produced by the cells in the joint cartilage), B cells (type B synovialocytes or F cells, active fibroblasts which produce and secrete the outer collagen and proteoglycan aggregates, i.e. hyaluronic acid of the synovia) and the subsynovial connective tissue rich in capillaries, fibroblasts, and lipocytes. Collagen fibres within the articular cartilage are arranged in arcades (BENNINGHOFF’s arcades).
Types of joints
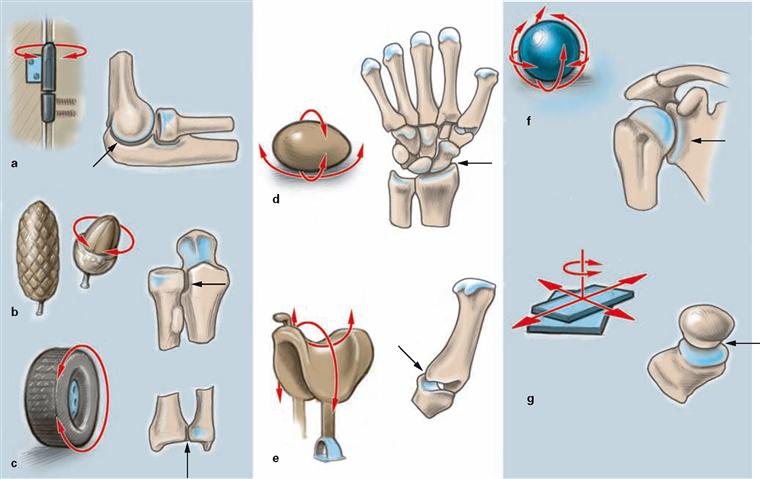
Figs. 1.24a to g Joints, Juncturae synoviales [Articulationes, Diarthroses].
Joints usually increase the range of motion significantly. They are classified according to the shape of their articulating surfaces and/or the freedom of movement they allow. Based on the main axes of motion, we distinguish uniaxial, biaxial, and multiaxial joints.
a hinge joint, Articulatio cylindrica (Ginglymus): uniaxial joint, permits flexion and extension
b conoid joint, Articulatio conoidea: uniaxial joint, permits rotational movement
c pivot joint, Articulatio trochoidea: uniaxial joint, permits rotational movement
d condylar joint, Articulatio ovoidea, Articulatio ellipsoidea:
biaxial joint, permits flexion, extension, abduction, adduction, and restricted rotational movement
e saddle joint, Articulatio sellaris: biaxial joint, permits flexion, extension, abduction, adduction, and restricted rotational movement
f spheroidal or ball and socket joint, Articulatio spheroidea: multiaxial joint, permits flexion, extension, abduction, adduction, and rotational movement
g plane joint, Articulatio plana: joint permits simple gliding movements in different directions
Range of joint movement
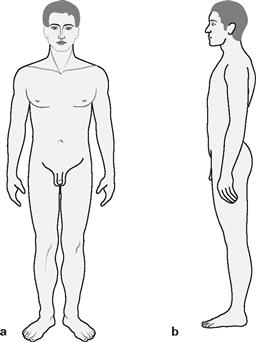
Figs. 1.25a and b Documentation of the range of joint movement: neutral-null method.
The neutral-null method is a standardized goniometric method to determine the active range of movement in a joint. An upright position with arms hanging down to each side is considered the zero degree starting position when examining the joints (a view from the front and b from the side). The extent of achievable movement from this null position is expressed in degrees of angle measured. First the active range of movement away from the body is determined, followed by the active range of movement towards the body.
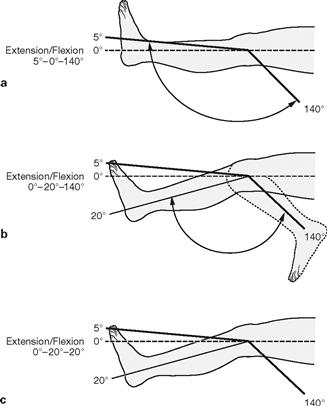
Figs. 1.26a to c Documentation of the range of joint movement: Examples.
a. The normal healthy knee joint has the following range of movement: 5° extension and 140° flexion (not shown). The 90° angle of the ankle joint in relation to the foot is considered the null position. This allows for a 20° extension and 40° flexion under normal conditions (not shown). The normal range of movement in the knee joint is 5°–0°–140° (knee stretched, null position, knee bent), that of the ankle joint is 20°–0°–40° (dorsal extension, null position, plantar flexion).
b. stretching of the knee impossible (see Clinical Remarks box)
c. complete stiffness of the knee (see Clinical Remarks box)
Types of muscles
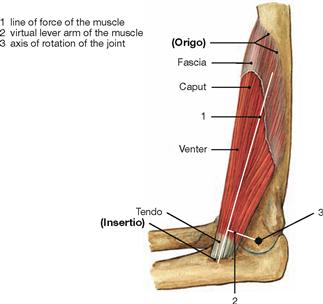
Fig. 1.27 Organization principle of skeletal muscles, exemplified by the brachial muscle, M. brachialis.
Skeletal muscles move bones in their joints and have a fixed point of origin (Origo) and a flexible point of insertion (Insertio). They are surrounded by a fascia. The belly of the muscle (Venter, Gaster) connects with the bone through a tendon. The amount of force a muscle can transfer onto a joint depends on the length of the lever (vertical distance of the vector force of the muscle and the rotational axis of the joint = lever arm of force). The length of the lever varies depending on the joint position and is known as virtual lever.
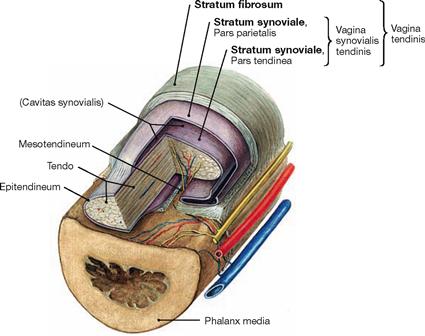
Fig. 1.28 Structure of a tendon sheath, Vagina tendinis, Vagina synovialis, exemplified by a finger.
Tendon sheaths reduce friction during movement and protect tendons which are deflected by muscles and bones. The composition of a tendon is similar to that of a joint capsule. The inner layer of the tendon sheath (Stratum synoviale, Pars tendinea) is part of the tendon, whereas the outer layer (Stratum synoviale, Pars parietalis) is part of the Stratum fibrosum of the tendon sheath. The gap between both layers (Cavitas synovialis) contains synovial fluid (Synovia).
Small blood vessels reach the tendon via Vincula brevia and longa (small ligaments from the mesotendineum).
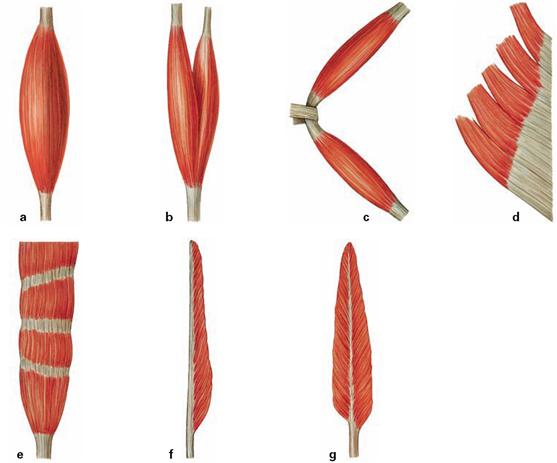
Figs. 1.29a to g Types of muscles. Microscopically, fibres of skeletal muscles exhibit typical cross-striations. Based on their shape skeletal muscles can be divided into:
a. single head, parallel muscle fibres (Musculus fusiformis)
b. double head, parallel muscle fibres (Musculus biceps)
c. double belly, parallel muscle fibres (Musculus biventer)
d. multi-head, flat muscle (Musculus planus)
e. multi-belly muscle with tendinous intersections (Musculus intersectus)
Muscle biomechanics
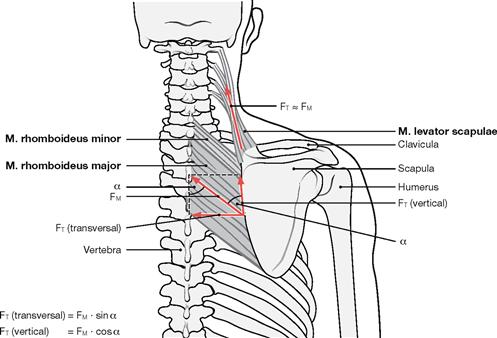
Fig. 1.30 Forces of muscles and tendons; vector forces of the muscles and tendons exemplified by the Mm. levator scapulae and rhomboidei. (according to [1])
There is a direct proportional relationship between the muscle force and the physiological cross-section of this muscle (lifting force of a muscle relative to the cross-section of all muscle fibres positioned perpendicular to the direction of these fibres). When the direction of a tendon and the vector force of the muscle align, the full force of the muscle is transferred to the tendon. In this case, muscle force (FM) and tendon force (FT) are almost equal. However, when the muscle fibres are oriented in an angle to the pull by the tendon (e.g. Mm. rhomboidei major and minor), only part of the contractile force is transferred to the tendon. Here the vertical tendon force (FT [vertical]) is reduced by the factor cos α and the transverse tendon force (FT [transverse]) is reduced by the factor sin α relative to the muscle force (FM).
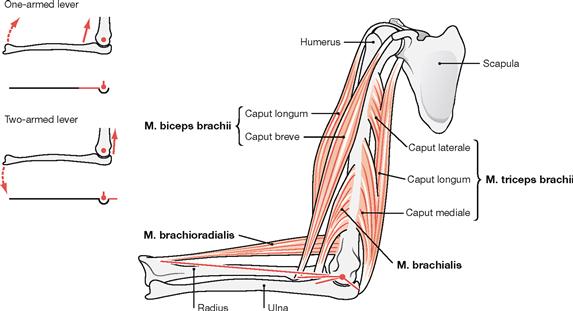
Fig. 1.31 Lever and muscle action; major muscles of the elbow joint and their anatomical levers (red lines). (according to [1])
The lever arm is the part of a lever which acts between the centre of rotation and the point where the force acts. For skeletal components to be moved around a rotational axis of a joint, a muscle must use an anatomical (existing) lever arm to create a torque. The length of the lever arm depends on the distance between the origin of a muscle and the centre of rotation of the joint. For example, when the arm is moved towards the torso the M. brachioradialis and the M. brachialis have a long and short anatomical lever arm, respectively. When muscle force is applied via an one-armed lever, the skeletal component will move in the direction of a traction force of this muscle (e.g. Mm. brachioradialis, biceps brachii, brachialis). With a two-armed lever, the point of muscle origin is moved in the direction of the muscular traction, but the main part of the skeletal component is moved in the opposite direction (e.g. M. triceps brachii; compare → Fig. 1.27).
The cardiovascular system
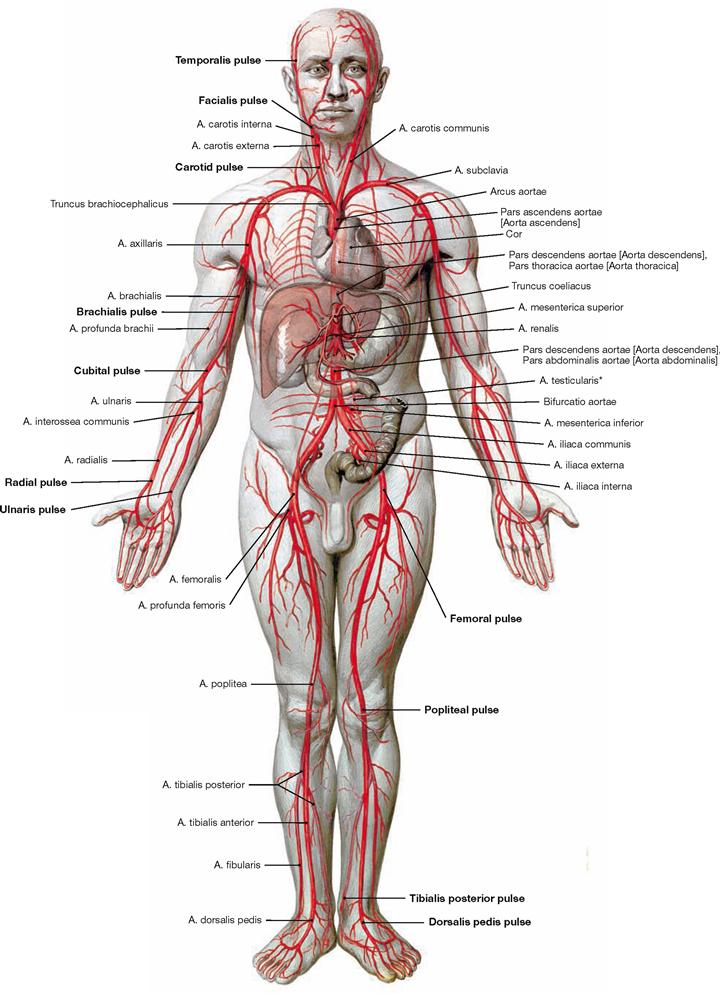
Fig. 1.32 Overview of the arteries of the systemic circulation.
The function of arteries is to transport blood from the heart to the periphery of the body or into the lungs. We distinguish arteries of the elastic type (e.g. aorta, arteries close to the heart) and arteries of the muscular type (most of the arteries, e.g. Aa. brachialis and femoralis). Blood travels through arteries with ever more narrow diameter to reach arterioles and enter into a capillary network where the exchange of oxygen takes place between the blood and the tissue.
* in women: A. ovarica
Vessels and nerves
The cardiovascular system
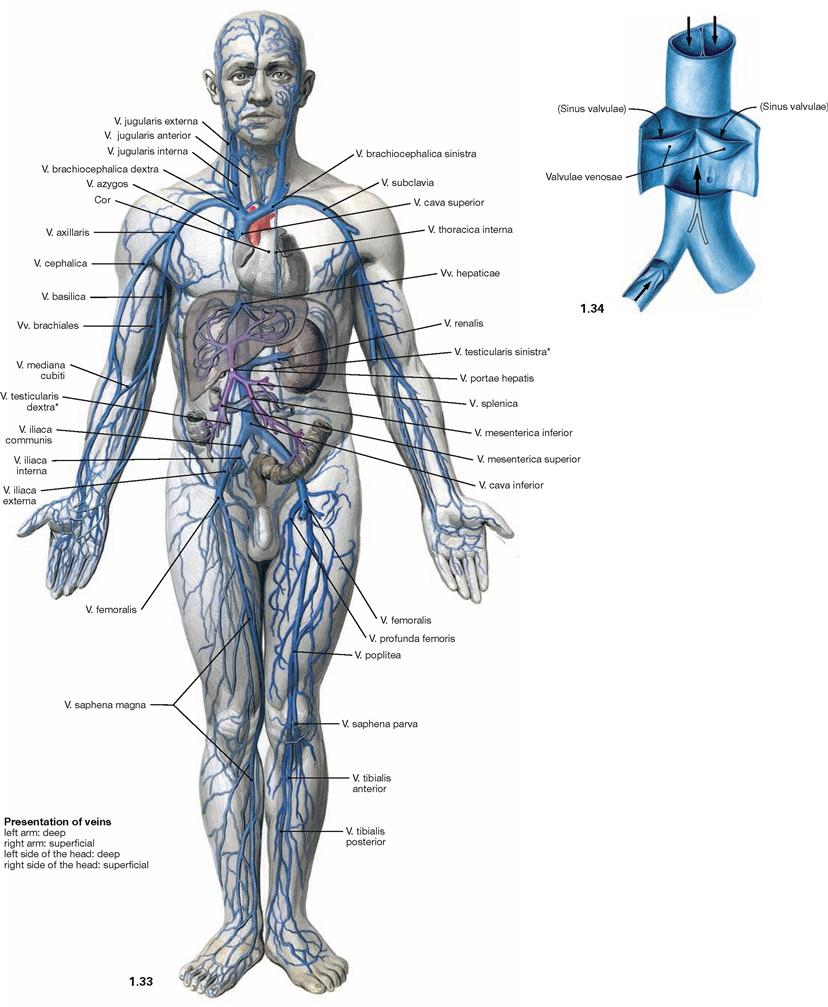
Fig. 1.33 and Fig. 1.34 Overview of the veins of the systemic circulation (→ Fig. 1.33) and venous valves (→ Fig. 1.34).
Veins transport blood from the periphery of the body back to the heart. They expand easily and function as reservoirs. The veins of the systemic circulation transport deoxygenated blood, those of the lung circulation transport oxygenated blood. Most veins are concomitant veins, meaning they run in parallel with corresponding arteries. Compared to the arteries, their course is variable and the blood pressure is significantly lower. Veins, capillaries, and venoles are part of the low pressure system of blood circulation. Most of the time, veins transport blood against gravitational force. Thus, larger veins of the extremities and the lower neck region possess valves (venous valves) to support the venous blood flow back to the heart. Apart from the valves, muscles and the arterial pulse (only when venous valves are present) also affect the venous blood flow.
Arrows pointing upwards indicate the direction of blood flow. When blood accumulates (arrows pointing downwards) the valves close.
Most parts of the body contain a superficial venous system in the subcutaneous fat pad which communicates with a deeper venous system running parallel to the arteries (both systems are separated by venous valves so that blood can only travel unidirectionally from the superficial to the deep veins).
* in women: V. ovarica
Systemic, pulmonary, and fetal blood circulation
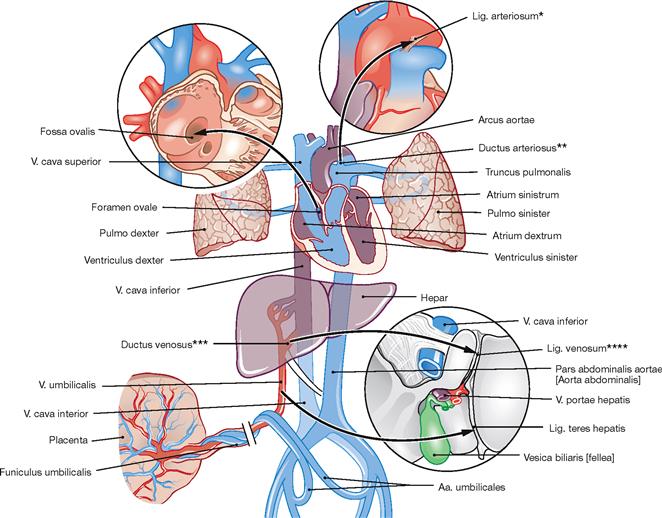
Fig. 1.35 The prenatal circulation; schematic representation. (according to [1])
Arrows indicate the direction of blood flow. The prenatal circulation is different from the circulation after birth.
Oxygenated blood is transported from the placenta and through the umbilical vein to the liver where most of the blood is drained by the Ductus venosus (ARANTII) directly into the V. cava inferior. From here, the major part of the blood reaches the right atrium of the heart, crosses over to the left atrium via the open Foramen ovale in the atrial septum, enters the left ventricle, and is ejected into the aorta and systemic circulation. Venous blood of the upper half of the body enters the right atrium through the V. cava superior and is directed mostly into the right ventricle. When the heart contracts, most of the ejection fraction is transported via the Ductus arteriosus (BOTALLI) directly into the Aorta descendens. Both shortcuts in the heart (open Foramen ovale and open Ductus arteriosus [BOTALLI]) are required since in the fetus the fluid-filled lungs are not yet inflated and constitute a barrier. Blood from the fetal systemic circulation is routed mainly via the internal iliac arteries (Aa. iliaca internae) into the paired umbilical arteries (Aa. umbilicales) located within the umbilical cord to reach the placenta. A sequence of events shortly after birth which involves the termination of the placental circulation, the inflation of the lungs, and the onset of breathing in the newborn results in the occlusion of:
At this point, the cardiovascular system only consists of the heart, the systemic circulation (body circulation; supply of body tissues, and the smaller pulmonary circulation (gas exchange) (→ Fig. 5.10). The ejection fraction of the heart of a resting adult is 70 ml.
Approximately 64% of blood resides in the venous system at any given moment and this can increase to approximately 80% (blood reservoir).
The small arteries and arterioles of the muscles mainly determine the vascular resistance. In the arterial system (high pressure system) the average blood pressure is approximately 100 mmHg (= mm mercury column), whereas in the venous system it is approximately 20 mmHg. Both systems are separated by the capillary bed where the exchanges of gas and nutrients take place.
* BOTALLO’s ligament
** BOTALLO’s duct
*** ARANTIUS’ duct
**** ARANTIUS’ ligament
Portal vein system
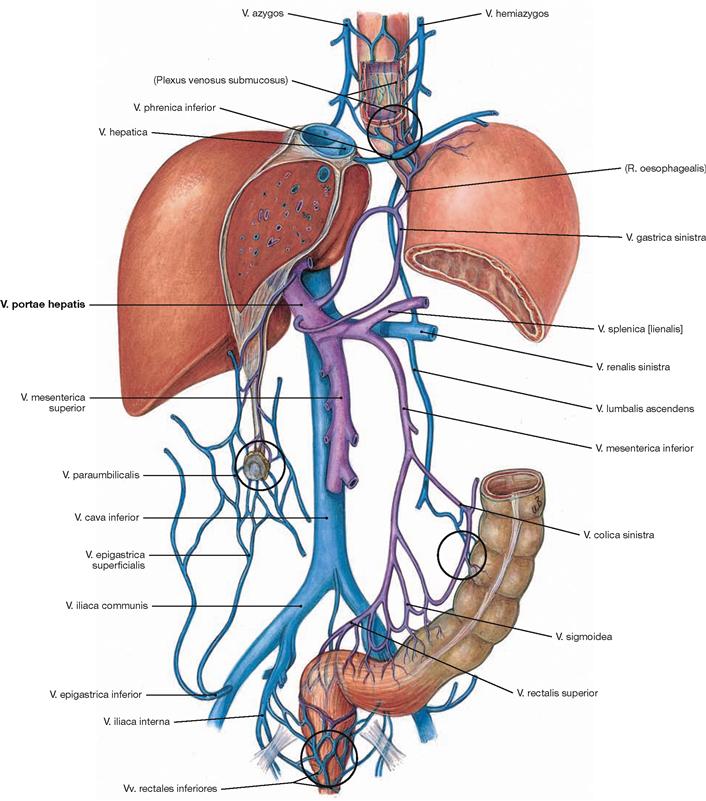
Fig. 1.36 Portal vein, V. portae hepatis, and inferior vena cava, V. cava inferior; semi-schematic representation; tributaries to inferior vena cava in blue; tributaries to the portal vein in purple. Potential portal-systemic anastomoses are encircled in black.
The portal-venous circulation constitutes a special part of the systemic circulation. Here, two separate capillary beds (intestine, liver) are connected in sequence. Prior to reaching the systemic circulation, venous blood from most unpaired abdominal organs (stomach, parts of the intestine, pancreas, spleen) is drained into the portal vein and from here into the liver. This way, most of the nutrients absorbed through the intestinal tract first reach the liver and are metabolized there. Not until the blood has passed the liver, is it drained via the liver veins (Vv. hepaticae) into the inferior vena cava and the systemic circulation.
Lymphatic system
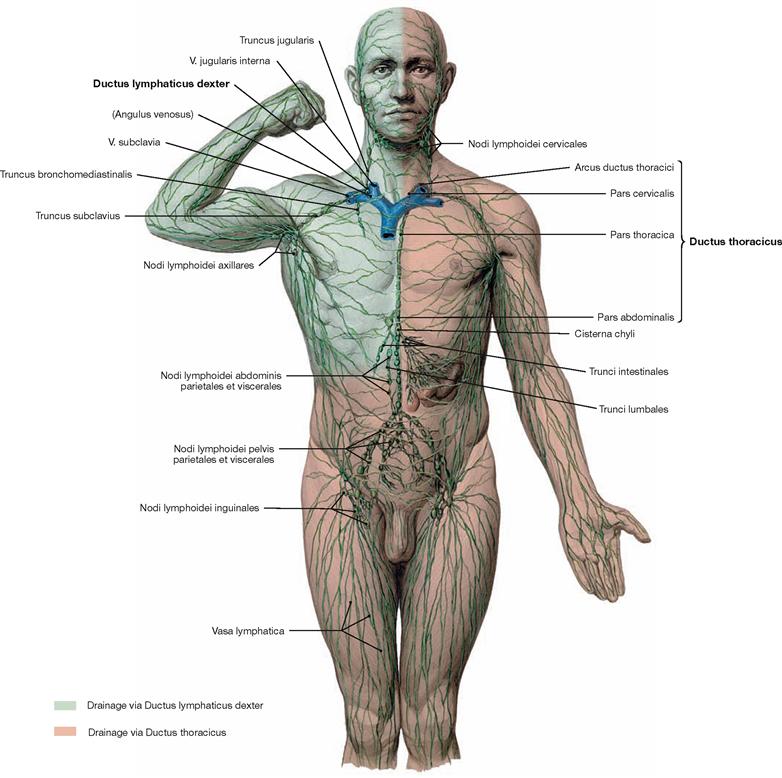
Fig. 1.37 Overview of the lymphatic system.
Starting in the body periphery, lymph capillaries collect interstitial fluid (lymph) and transport it via collecting ducts into lymph vessels and lymph nodes. Lymph nodes responsible for the collection and filtration of a particular body region are called regional lymph nodes. Those lymph nodes accepting lymph fluid from different lymph nodes are called collector lymph nodes.
Finally, the lymph reaches two major lymphatic ducts, the Ductus thoracicus and Ductus lymphaticus dexter, which drain the lymph into the venous blood of the systemic circulation. The major part of the lymph drains into the left venous angle (Angulus venosus, located between V. jugularis interna sinistra and V. subclavia sinistra) via the Ductus thoracicus. The Ductus lymphaticus dexter drains the lymph collected from the right upper quadrant into the right venous angle (located between V. jugularis interna dextra and V. subclavia dextra).
In addition to the lymph vessels and lymph nodes the lymphoid tissue also includes lymphatic organs (thymus, bone marrow, spleen, tonsils, mucosa-associated lymphoid tissue [MALT]). The lymphatic system has important functions in immune responses and resorption of lipids.
Lymph nodes
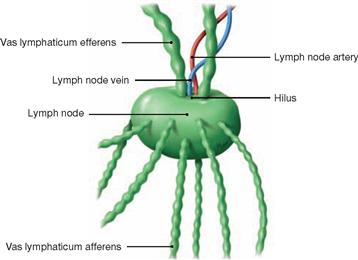
Fig. 1.38 Lymph nodes with in- and outgoing lymph vessels; semi-schematic representation.
Lymph nodes are part of the lymphatic system and considered secundary lymphatic organs. They come in various shapes (mostly lens- or bean-shaped with a diameter of 5–20 mm). The body contains about 1,000 lymph nodes and of those 200 to 300 are located in the neck alone. Functionally, lymph nodes are part of the immune system and play an important role in the defence against infections.
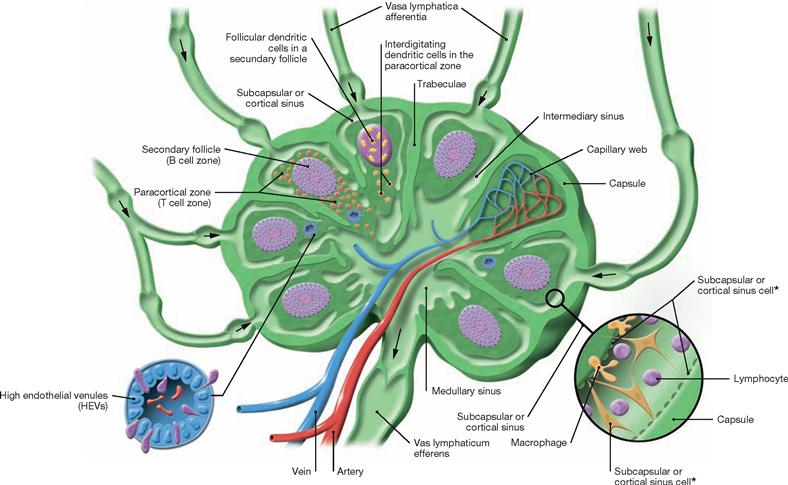
Fig. 1.39 Lymph nodes; schematic cross-section. (according to [2])
This cross-section of a representative lymph node shows in- and outgoing lymph vessels (Vasa afferentia and Vasa efferentia), blood supply, and compartmentalization of the lymph node into B region (secondary follicle), T region (paracortical zone) with postcapillary or high endothelial venules, follicular and interdigitating dendritic cells, medullary sinus, intermediate sinus, and subcapsular or cortical sinus (with cellular composition shown).
* Reticular cells lining the sinus wall also reside within the sinus.
Nervous system
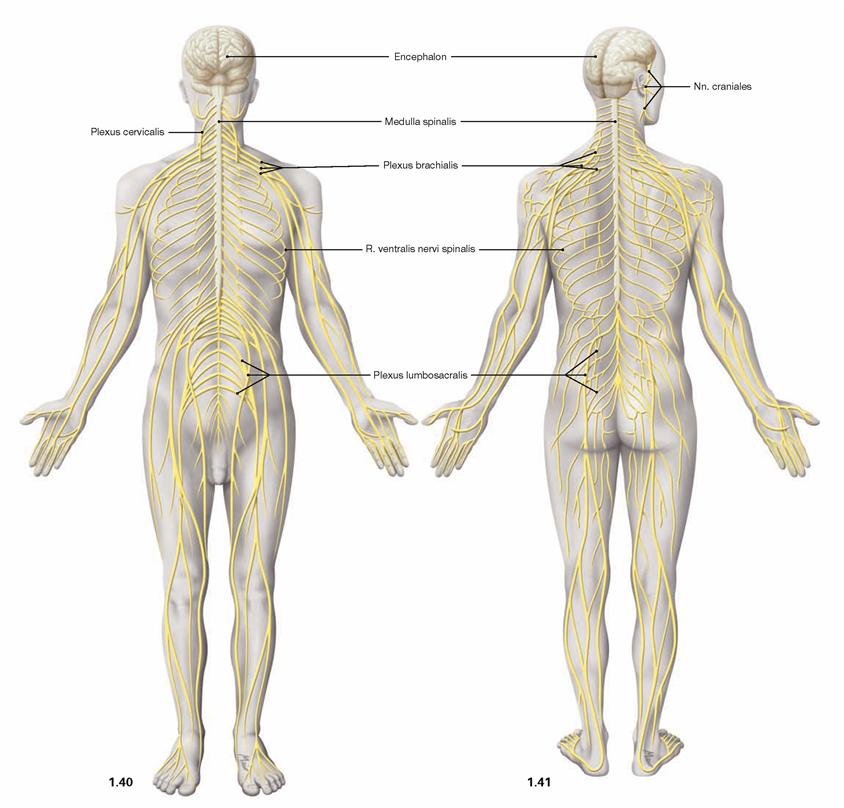
Fig. 1.40 and Fig. 1.41 Organization of the nervous system;
ventral (→ Fig. 1.40) and dorsal view (→ Fig. 1.41). (according to [2])
The nervous system is composed of the central (CNS; brain, spinal cord) and peripheral nervous system (PNS). The PNS is mainly com-posed of spinal nerves (with connections to the spinal cord) and cranial nerves (with connections to the brain).
The nervous system is involved in complex functions that include the regulation of the activities of the muscles and the intestines, the communication with the environment and the inner self, and memorizing past experiences (memory). The nervous system is also essential for conceptualizing imaginations (thinking), generating emotions, and adapting quickly to changes in the surrounding world and the body interior. We distinguish the autonomic (visceral, regulating the activities of the intestines, predominantly involuntary) and the somatic (innervation of skeletal muscles, cognitive perception of sensory input) nervous system. Both systems interact with and affect each other. Apart from the nervous system, overall body functions are also regulated by the endocrine system.
Spinal nerves
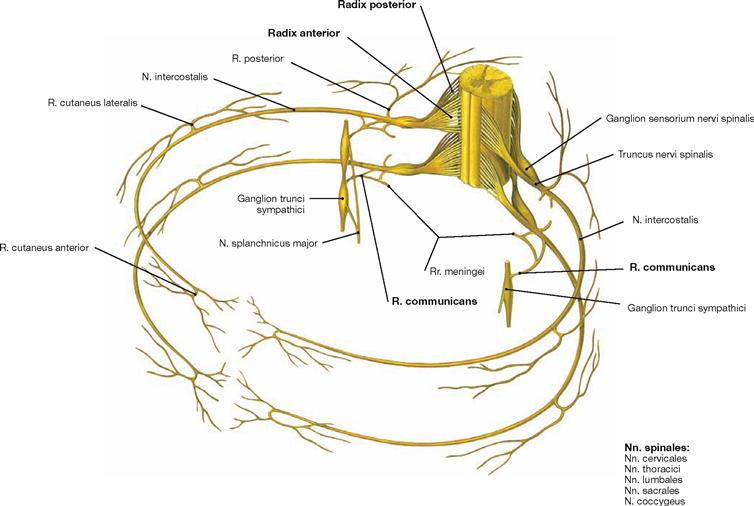
Fig. 1.42 Schematic representation of a spinal nerve (spinal cord segment) exemplified by two thoracic nerves; view from above in an oblique lateral angle.
The human body has 31 pairs of spinal nerves (eight cervical, twelve thoracic, five lumbar, five sacral pairs, and one coccygeal pair). Each spinal nerve is composed of an anterior root (Radix anterior) and a dorsal root (Radix posterior). The cell bodies (Perikarya) of motor nerves are located in the grey matter within the spinal cord. Their axons leave the spinal cord forming the anterior root. The perikarya of sensory nerves are located in the dorsal root ganglia (Ganglia sensoria nervi spinalis). Their processes enter the spinal cord via the dorsal roots. Rami communicantes connect the spinal cord with the sympathetic chain of ganglia (Ganglia trunci sympathici) of the sympathetic trunk (Truncus sympathicus). All branches of the dorsal spinal nerves as well as the ventral branches of the thoracic spinal nerves T2 to T11 have a segmental arrangement. The other ventral branches converge to form plexus (Plexus cervicalis, brachialis, lumbosacralis).
Autonomic nervous system
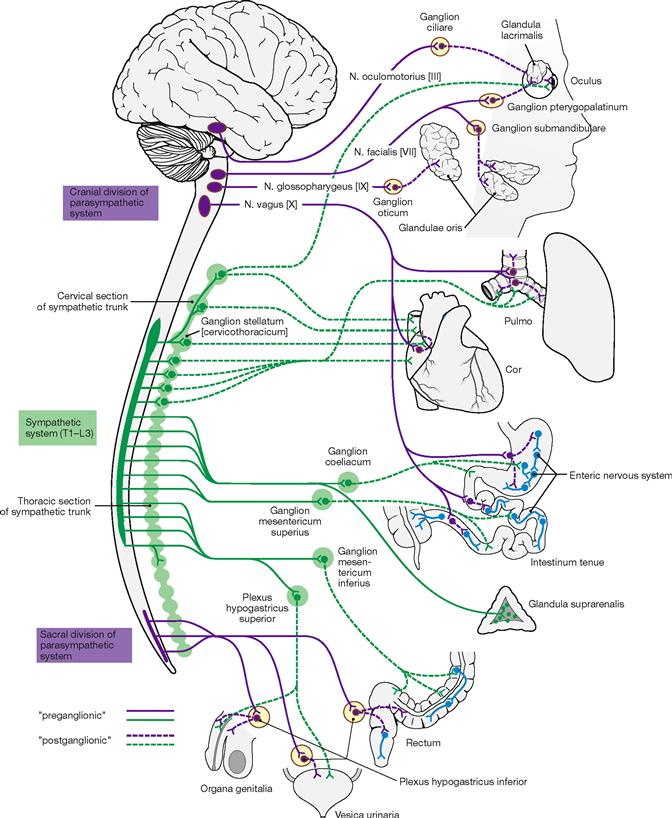
Fig. 1.43 Autonomic nervous system. [22]
The autonomic nervous system consists of the sympathicus, parasympathetic (Parasympathicus and enteric nervous system.
The nerve cells of the Sympathicus are located in the lateral horn of the thoracolumbar segment of the spinal cord. Their axons project into the ganglia of the sympathetic chain and the ganglia of the gastro-intestinal tract. Here, the preganglionic fibres synapse on postganglionic neurons which send their processes to the target organ. Activation of the symphatetic system occurs during mobilization of the body and in emergency situations (the three Fs: fright, flight, fight). The medulla of the adrenal gland is part of the sympathetic system and secretes adrenaline (epinephrine) and noradrenaline (norepinephrine) into the circulation.
The nuclei of the Parasympathicus are located in the brain stem and the sacral spinal cord. Preganglionic parasympathetic axons reach ganglia close to their target organs. Here, they synapse with postganglionic neurons which send short axons to their target organs. The parasympathetic system regulates food intake and digestion as well as sexual arousal. The Parasympathicus is the antagonist of the Sympathicus.
The enteric nervous system regulates the activity of the intestinal tract and is controlled by Sympathicus and Parasympathicus.
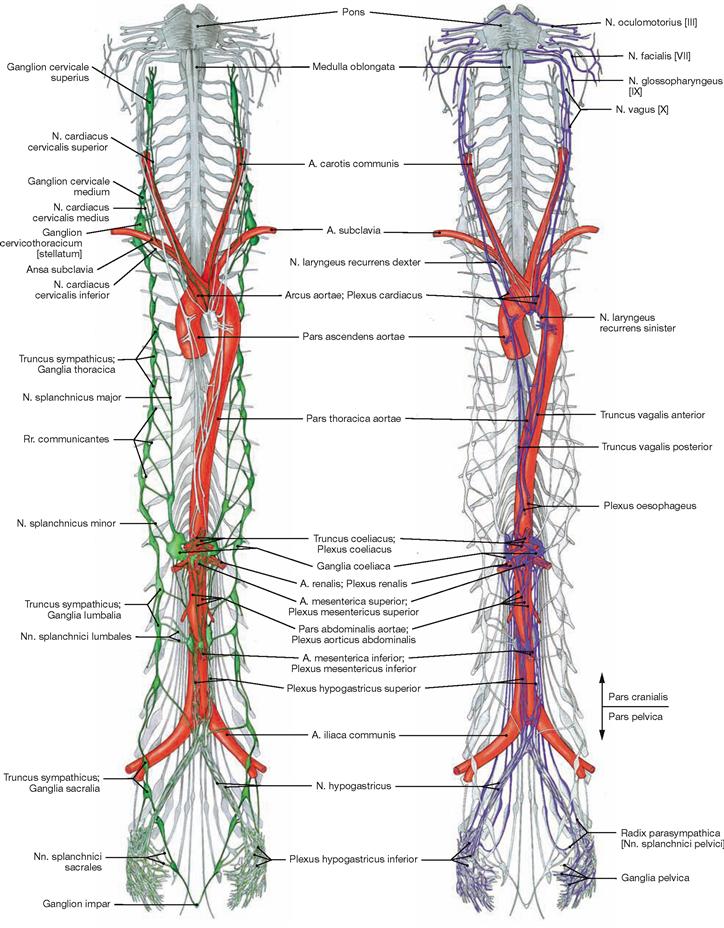
Fig. 1.44 Representation of the Sympathicus, Pars sympathica.
The entire sympathetic chain of ganglia and their interganglionic connections located to both sides of the vertebral column are called the Truncus sympathicus (green).
Radiography, fluoroscopy
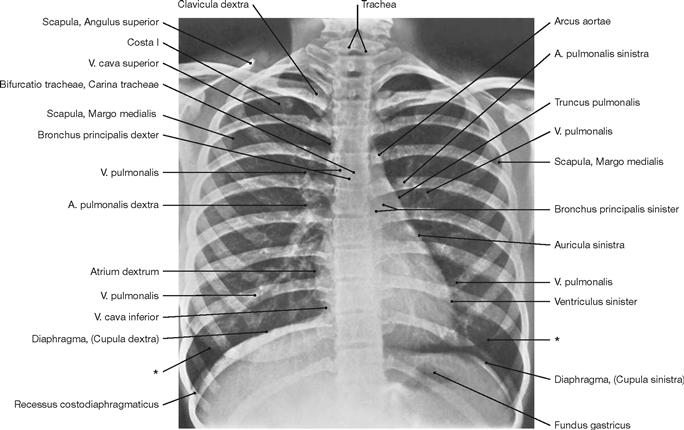
Fig. 1.46 Conventional radiograph (X-ray), overview of the thorax. [27]
Radiography is one of the most frequently used imaging techniques in hospitals and local clinical practice. Familiarity with the imaging technique is essential in understanding how such images are being generated and what type of radiographic image is viewed. Simple radiographic images of the thorax are among those most frequently generated. With a patient standing upright, the X-rays pass through the thorax in a posterior-anterior (PA) direction (patient faces radiographic film). In the lying position, the X-rays pass through the patient in an anterior-posterior (AP) direction. A good radiographic image of the thorax displays the major bronchi and blood vessels of the lung, the cardiomediastinal conture, the diaphragm, the ribs, and the peripheral soft tissue.
* conture of the breast (mamma)
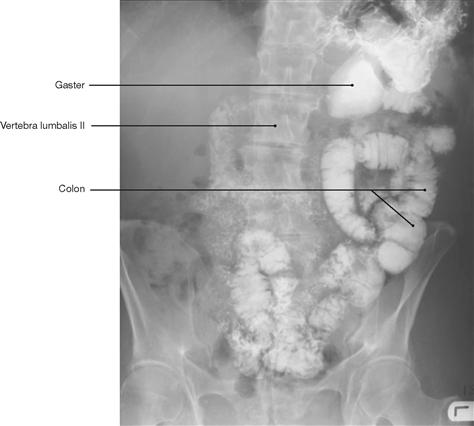
Fig. 1.47 Conventional radiograph (X-ray), colon fluoroscopy after barium swallow test. [8]
In a radiograph, hollow organs, such as arteries, veins, and intestinal loops, are poor in contrast and need to be filled with a substance that absorbs X-rays to increase contrast. These substances must not be toxic to the patient. A frequently used substance to increase contrast of the gastro-intestinal tract is the insoluble, non-toxic, high density salt barium sulfate. For applications in vessels iodine-containing molecules are usually employed. These substances are safe and well tolerated by most patients and can also be used to image the kidneys, ureters, and bladder (intravenous urogram [IVU], intravenous pyelogram [IVP]) as they are excreted by the kidneys.
Imaging techniques
Scintigraphy and ultrasound
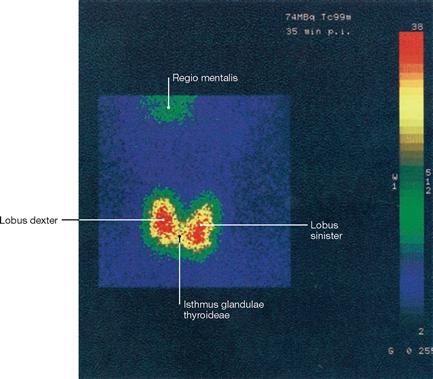
Fig. 1.48 Scintigraphy, scintigram of the thyroid gland. [27]
In scintigraphy, gamma rays (a form of electromagnetic rays) are used to generate an image. Gamma rays are produced as a result of the decay of unstable atomic nuclei, whereas X-rays are excess energy released during the bombardment of atoms with electrons. The gamma ray emitter has to be administered to the patient. The radio-isotope technetium-99m (99mTc) is most frequently used and injected as a cocktail together with other molecules. Upon injection, images are generated by a gamma camera, depending on how the radiopharmacon is absorbed, distributed, metabolized, or excreted by the body.
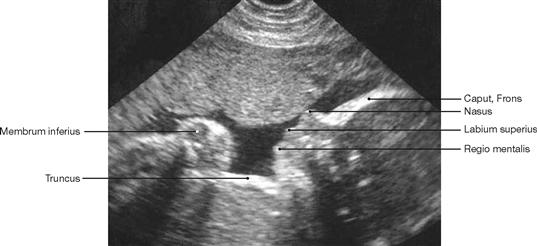
Fig. 1.49 Sonography, ultrasound image of a fetus at week 28 of pregnancy; lateral view.
Examinations of the body employing ultrasound are common in all medical specialties. Ultrasound represents a series of high-frequency sound waves (not an electromagnetic beam) generated by electric impulses in piezo-electric crystals. These sound waves are reflected from inner organs and their content (fetus in the uterus), registered by the same piezo-electric element, and transformed back into electrical impulses by the crystal. This information is then analysed by a computer and presented on a screen. This way, the movements of the extremities of the fetus and the opening of the mouth can be viewed as a live image.
Computed tomography (CT) and 3-D CT angiography
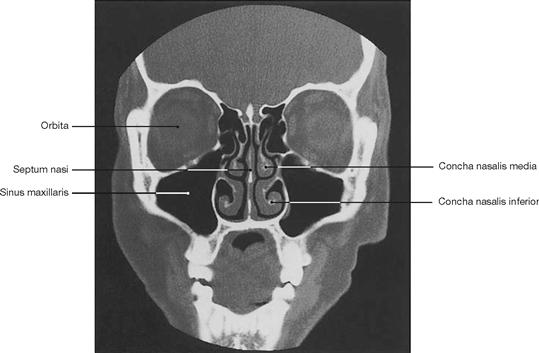
Fig. 1.50 Computed tomography, coronal computed tomogram (CT) of the sinuses. [11]
Computed tomography (CT) was developed by Sir Godfrey Hounsfield in the 1970es and has undergone constant refinement. The computed tomograph generates a series of sectional images through the body in the transverse or, as shown here, the coronal plane. The patient rests on a table and, while circulating the body, an X-ray tube takes one sectional image after the other. Once all images have been acquired, the individual sectional images are calculated by a computer applying complex mathematical algorithms.
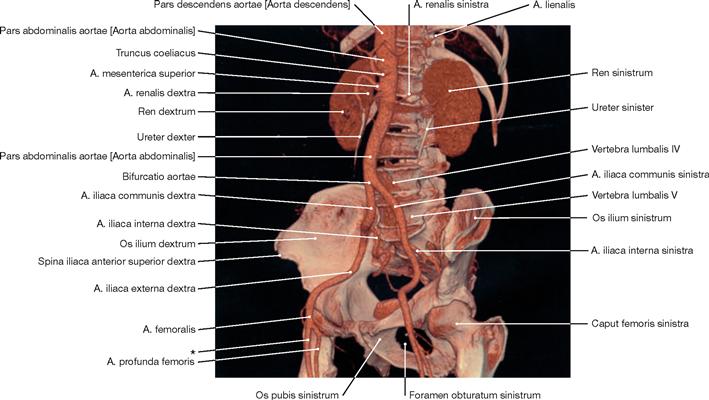
Fig. 1.51 3-D CT angiography, 3-D CT angiogram of different structures of the abdomen and pelvis (volume-rendering technique, VRT) derived from multidetector CT sections. [27]
Modern computed tomography technology (e.g. 64-lines volume spiral multilayer CT) provides new dimensions and indications for CT diagnostics and guarantees minimal dosage exposure for patients.
CT angiography is based on the same multilayer CT technology. In a blood vessel, the region of interest is scanned during fast intravenous injection of a iodine-containing substance to increase contrast of the structure. The resulting sectional images of branching vessels are then assembled by a computer to generate a 3-D image.
* clinical term: A. femoralis superficialis
Magnetic resonance imaging (MRI)
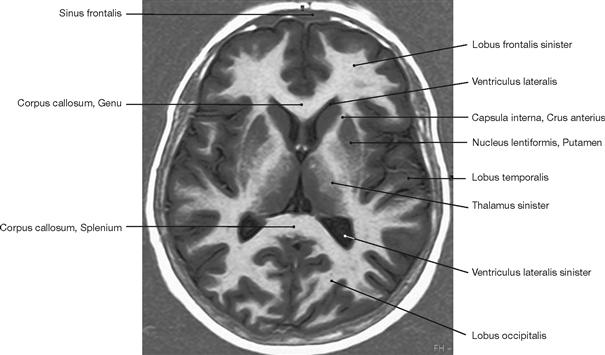
Fig. 1.52 Magnetic resonance tomography (MRT) or imaging (MRI), axial (transverse) magnetic resonance image of the brain (T2-weighted). [27]
In magnetic resonance imaging patients are exposed to a powerful magnetic field. This causes all protons of hydrogen atoms in the body to align with the magnetic field which effectively transforms these hydrogen protons to become miniature magnets. Then patients are briefly exposed to radiofrequency pulses to systematically change the alignment of these protons.
When returning to their original position, the protons emit a weak radio wave that is detected by the instrument. The strength, frequency, and time it takes for the protons to return to their original position is an important information contained within the emitted signal and analysed by a computer to generate an image.
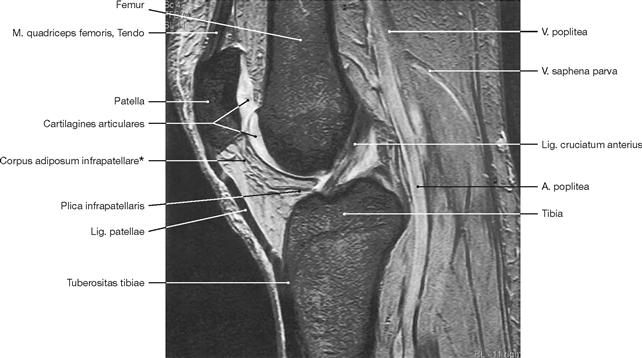
Fig. 1.53 Magnetic resonance tomography, sagittal magnetic resonance image (MRI) of a knee (T2-weighted). [27]
Altering the sequence of impulses used to excite protons allows for different characteristic features of these protons to be analysed. These characteristics are called “weighting” of an MRI scan. Alterations in pulse frequency and scanning parameters result in T1-weighted (fluids: dark, fat: bright; e.g. joint effusion dark) and T2-weighted (fluids: bright, fat: grey; e.g. the conspicuous HOFFA’s fat pad between the patella and tibia) images. Thus, specifically T-weighted images emphasise particular tissue compartments. MRI can also be used to generate angiograms of the peripheral and central circulation.
* HOFFA’s fat pad
Nails
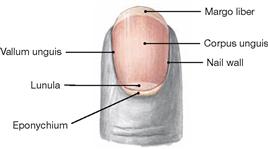
Fig. 1.54 Distal finger phalanx with nail.
The nail (Unguis) is a convex-shaped, translucent keratin plate on the upper side of the distal phalanx of the finger and toe. It serves to protect the tips of the fingers and toes and supports the grasping function of the fingers. The nail embeds into cutaneous slits (nail grooves, Vallum unguis) and its lateral margin is covered by the cutaneous nail wall or fold on both sides of the nail. The epithelial layer extending from the nail wall at the base of the nail onto the dorsal nail plate is called eponychium. The nail plate is anchored here to the nail bed, the skin beneath the nail plate.
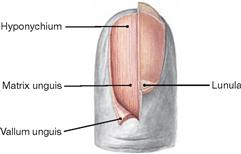
Fig. 1.55 Distal finger phalanx; nail partially removed.
The epithelium located beneath the free margin of the nail at the tips of the phalanges is called hyponychium (also known as “quick”). Beneath the epithelial hyponychium lies the fibrous base of the nail bed which is tightly connected with the periost of the distal phalanx. The proximal hyponychium forms the nail matrix (Matrix unguis) which generates the nail plate. The Lunula is the visible part of the nail matrix.
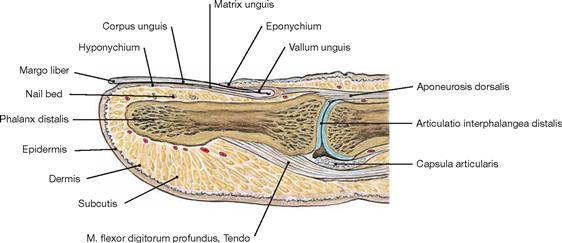
Fig. 1.56 Distal finger phalanx; Phalanx distalis; sagittal section.
The nail bed comprises the region between the nail and the distal phalanx. It consists of epithelium (Hyponychium and Matrix unguis) and the underlying dermis.
Integumentary system
Skin
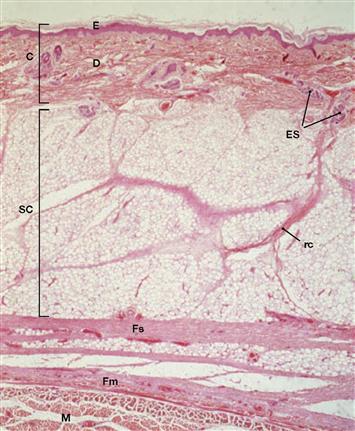
Fig. 1.57 Skin layers, Integumentum commune; (hairy skin);
C: cutis, composed of epidermis (E) and dermis (D); SC: subcutis; Fs: superficial fascia; Fm: muscle fascia; M: muscle; rc: Retinaculum cutis; ES: eccrine sweat glands. HE-staining, magnification: 22-fold. [2]
The skin (cutis) is composed of the epidermis and underlying dermis (fibro-elastic connective tissue with capillary plexus, specialized receptors, nerves, immune cells, melatonin-producing cells, sweat glands, hair follicles, sebaceous glands, smooth muscle cells; thickness varies depending on the body region). Beneath the dermis lies the subcutis (subcutaneous fat tissue). The skin is the largest organ of the body (approx. 2 m2) and serves many functions: it protects against mechanical injury, is a thermoregulator and a sensory organ, and prevents exces-sive fluid loss.
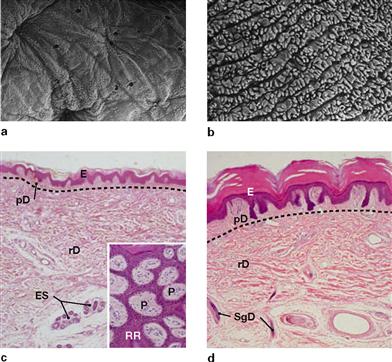
Figs. 1.58a to d Hairy skin (a and c, back of finger) and hairless skin (b and d, finger tip);
E: epidermis; P: papillae; pD and rD: papillary and reticular dermis; RR: rete ridges; ES: eccrine sweat glands; SgD: sweat gland efferent duct. The dotted lines indicate the margins between the dermal layers (Stratum papillare and Stratum reticulare). HE-staining. Magnification: 45-fold, inset 100-fold. (c, d [2])
The top panel shows scanning electron microscopy images of the surface of the dermal Stratum papillare after the epidermis has been digested and removed. The bottom panel shows the corresponding histological schematic overview images of sagittal sections through epidermis and dermis. The insert on the left image displays a tangential cut through the epidermis (purple) and the papillary dermis (pink).
Hair
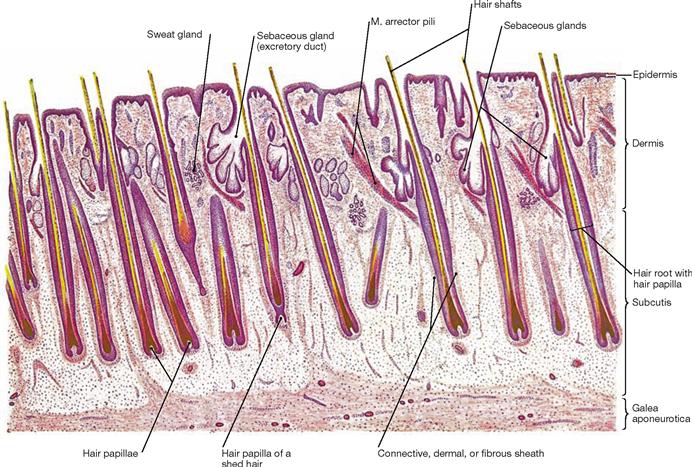
Fig. 1.59 Hairs, Pili; longitudinal section through the human scalp [24]
Hairs are the products of keratinization of the epidermis. They originate from invaginations of the epidermis which form follicles that contain mitotically active cells (matrix cells) at the base.
Matrix cells differentiate to become keratinized cells which form the shaft of the hair. Postnatally, we distinguish two types of hair:
• Vellus hair (fluffy hair) is soft, short, thin, largely without pigmentation, and does not contain a medulla (follicles are located in the dermis); similar to fetal lanugo hair, vellus hair covers most of the body in children and women.
• Terminal hair (long hair) is firm, long, thick, pigmented, and contains a medulla (follicles reach into the subcutis); it constitutes the hair of the head, eye brows, pubic region, arm pits, and beard (in men). The body distribution of terminal hair differs among ethnic groups.
Hair protects from UV-light and cold and serves to convey sensations of touch.
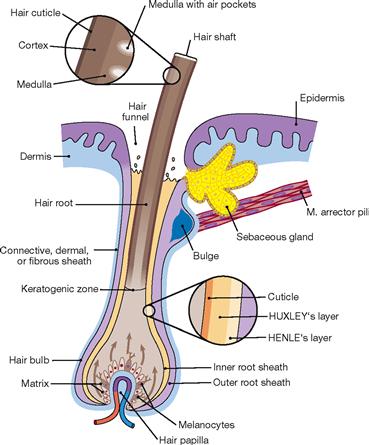
Fig. 1.60 Structure of a hair follicle; longitudinal section. [25]
Hair originates from hair follicles which are cylindrical invaginations of the epidermis into the dermis or subcutis. The hair follicular body consists of a hair bulb and the hair papilla. Each hair follicle receives a tuft of blood vessels to sustain its growth and is associated with a sebaceous gland (hair-sebaceous gland unit) and a smooth muscle (M. arrector pili). The latter is responsible for the erection of the hair (sympathetic activation) by indenting the epidermis to form small pits (goose bumps).
The following structures can be identified in a hair:
• a fully keratinized hair shaft with the epithelial inner and outer root sheaths
• non-keratinized hair root separated by the keratogenous zone (hair cells keratinizing) from the keratinized hair shaft
• hair bulb with its expanded base contains mitotically active matrix cells (regenerative part of the hair)
• dermal hair papilla, the cell- and blood vessel-rich dermal part which invaginates into the hair bulb from beneath
• hair infundibulum represents the surface opening of the follicle and contains the pilosebaceous canal of the hair-sebaceous gland unit
• epithelial root sheath of the hair which is divided into an inner and outer root sheath: cellular layers of the inner root sheath are (from hair medulla outward): cuticle, HUXLEY’s and HENLE’s layers; the outer root sheath is composed of multiple layers of bright, nonkeratinized cells which begin to keratinize in the infundibular region of the hair and integrate into the epidermis.
Genetic predisposition and pigmentation (melanin content) determine the hair colour. Once the production of melanin ceases, the hair turns from grey to white.